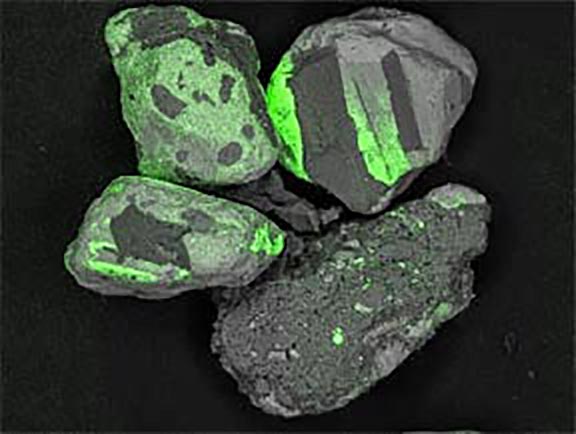
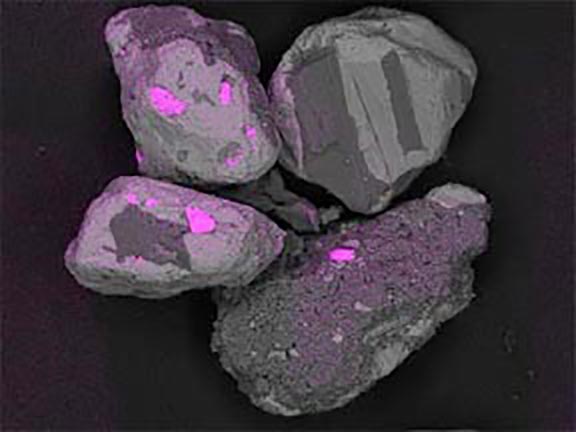
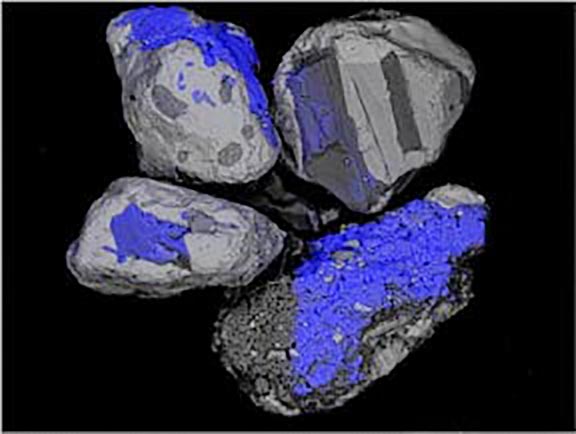
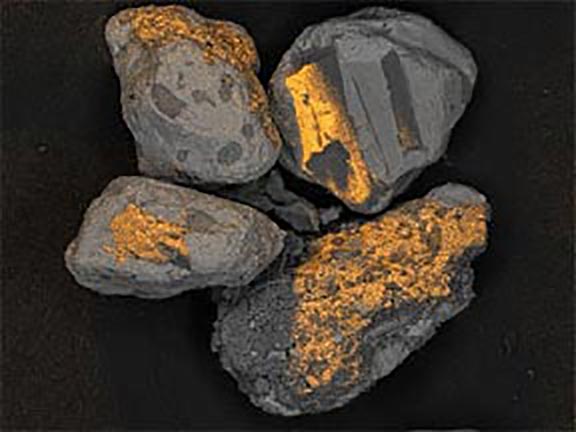Where did these minerals come from?
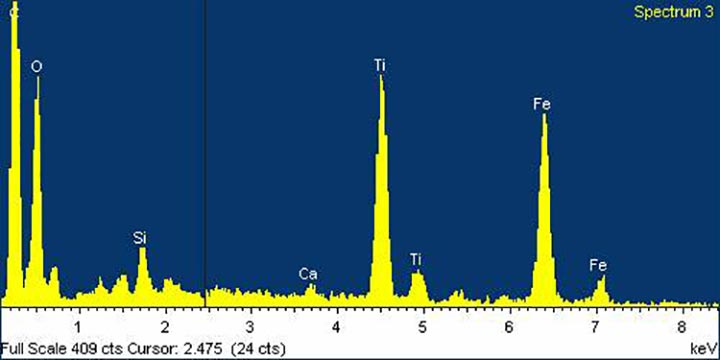
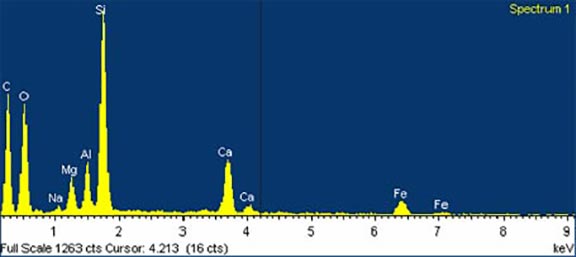
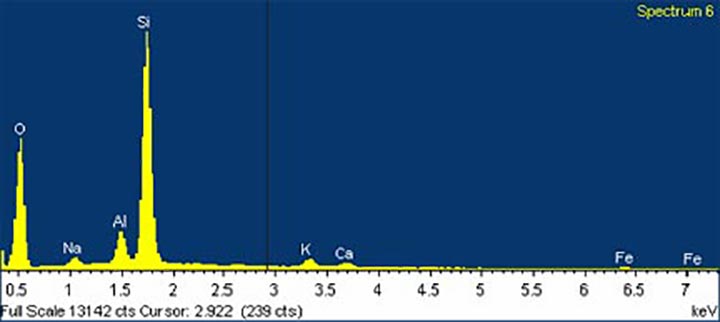
The exact origins of these sands are not clear cut. The dense ilmenite, a placer deposit, probably came from Coast Range mountains, from the basalt and other igneous rocks of ancient volcanic seamounts that make up the backbone of the Coast Range (Komar 1998, Orr and Orr 2000, Bishop 2003, Robert C. Witter, pers. comm.). Our local basalts are Grande Ronde basalts, massive layers of lava that originally flowed westward from eastern Washington and Oregon 15 to 18 million years ago and here formed Cape Meares, Cape Lookout, Three Arch Rocks, and other nearby sea stacks and cliffs. According to Dr. Stephen Reidel of PNNL and Washington State University (pers. comm.), these basalts contain titanomagnetite, but no pure ilmenite, so they are an unlikely source of this mineral. Other minerals in the sand, including augite and diopside, may also come from Coast Range basalt flows or marine sedimentary rock derived from eroded basic igneous rocks common in our area. Augite crystals can be found in the coast range. Some of the minerals may have come from sediments washed out of the Columbia River thousands of years ago. Some may have even come from the Klamath Mountains in southern Oregon and northern California (Bostrom and Komar 1997, Komar 1997, Paul Komar, pers. comm.).
Beach sand is moved by near-shore, wave-generated currents (Komar 1998). Much of the sand now washing out of Columbia River is deposited by these currents northward along the Washington coast. It is interesting to note that what sand washes south gets only as far as Tillimook Head, which serves as a barrier to any further southerly transport. It is important to note that, like Tillimook Head, the rocky seaward extensions of Cape Meares and Cape Lookout in our area currently form barriers to alongshore transport of sediments. The stretch of shore between these headlands is called a littoral cell, a kind of self contained region where sediment transports are more local. There are numerous littoral cells along Oregon that restrict coastal sand movement north or south.
These present headlands did not function as barriers during the ice ages. As Paul Komar, Professor Emeritus, Oregon State University, relates in his book “The Pacific Northwest Coast: Living With the Shores of Oregon and Washington (1998)”, during the ice ages a large amount of water was locked up in ice sheets and glaciers, and the level of the sea was lower than today’s sea level by about 125 meters (410 feet). This lowered sea level exposed vast areas of the continental shelf. The coastline, at that time located to the west of the current shore almost to the limit of the continental shelf, had a contour that probably conformed to the edge of the shelf, but the land on the shelf plane was fairly level and lacked headland barriers. Rivers draining the coastal mountains left sediments on this plane. Sands washed over the plane from the Klamath Mountains to the south of us, sediments flowing out of the Columbia River were deposited on the exposed landscape, and streams and rivers draining the coastal mountains dropped their loads of sediment. At the end of the last ice age, when the glaciers melted and their water was released back into the sea, the rising sea level allowed waves to move sediments up and down the coast, mixing them. As the sea finally rose to its present level and the headlands again became transport barriers, waves pushed the mixed sands shoreward onto the beaches of the littoral cells (Komar per. comm.). The sands from these distant sources, as a result of alongshore transport thousands of years ago, are the sands we find today on our beaches.
How do the sands sort into layers?
Two features of the different sands cause them to separate into the streaks and layers you see in Figure 1 and on the beaches between the Capes: density and grain size. I mentioned earlier that the density of blue-black sand, measured by the middle school student, was about twice that of the tan sand. To confirm this, you can find a deep deposit of ilmenite sand on the upper beach in Netarts. Take a handful of the ilmenite sand and compare its weight to a handful of the tan sand. You will see that the ilmenite sand is much heavier.
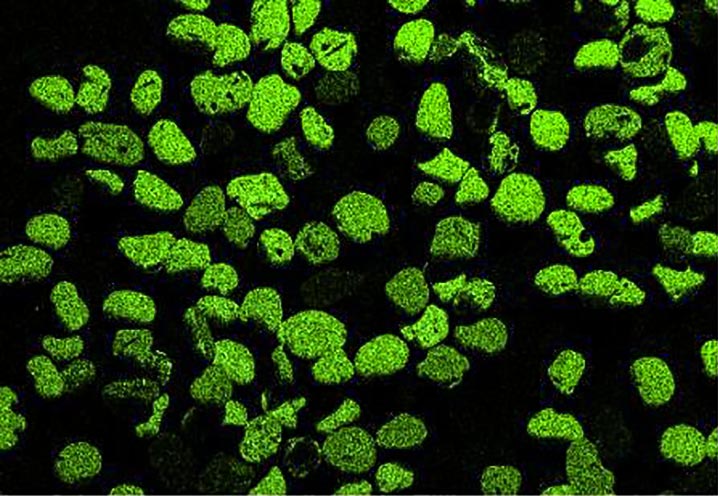
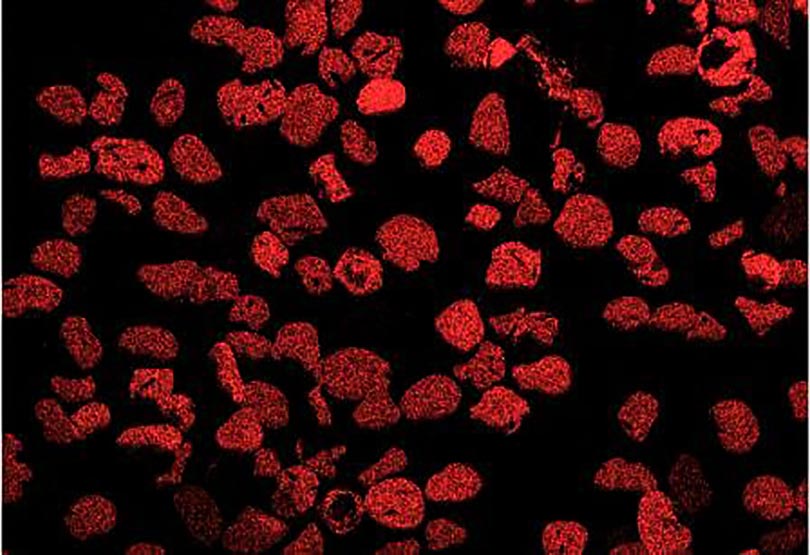
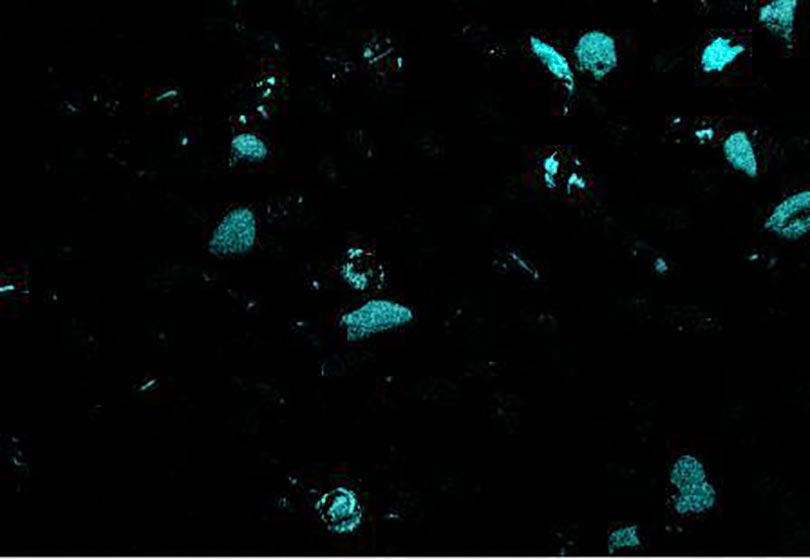
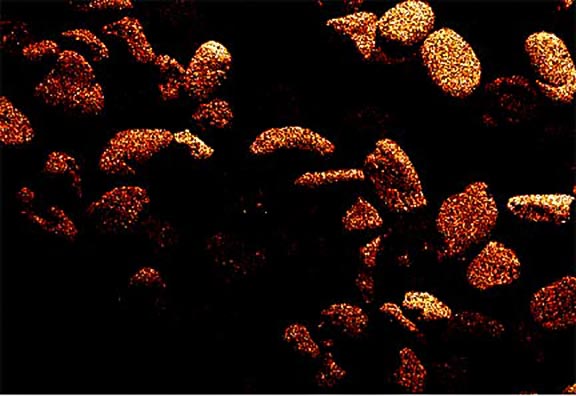
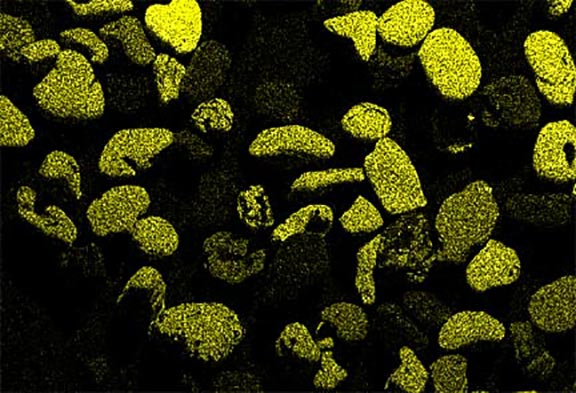
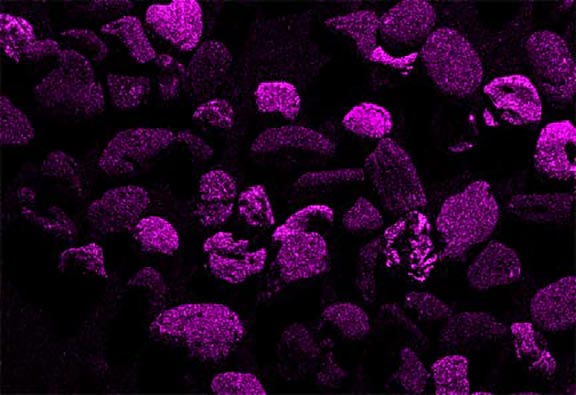
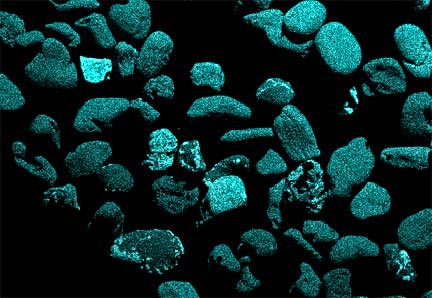
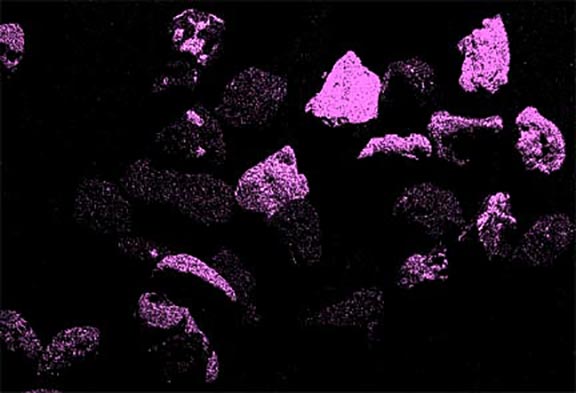
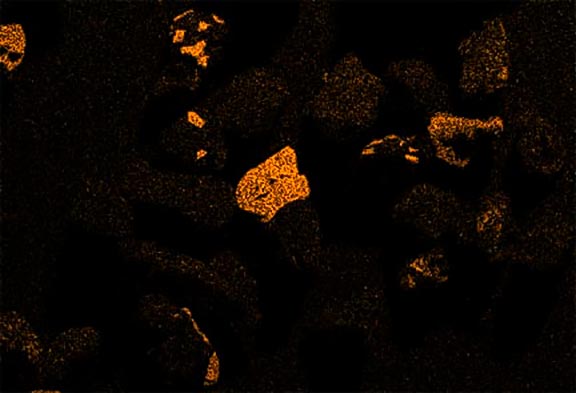
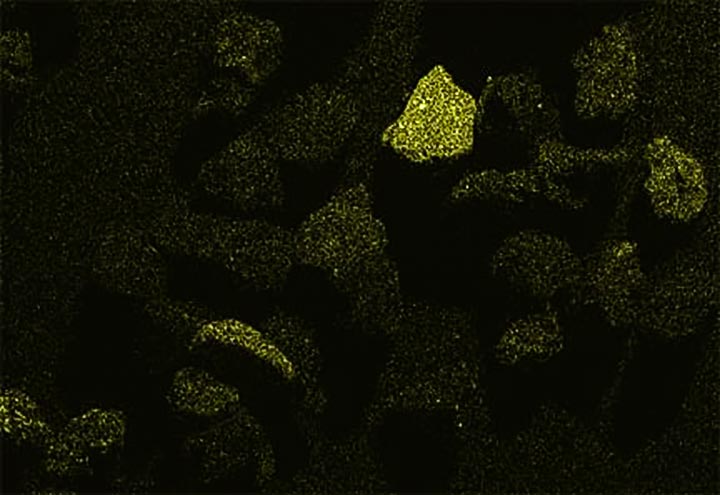
Now look at the micrographs (images) of the three sands taken by the SEM. You can see that the ilmenite has the smallest grains, the tan quartz and feldspars have the largest grains, and the grains of the brownish sands lie in between.
Paul Komar (pers. comm.) found that the low density and large size of the quartz and feldspar grains allowed them to be easily picked up by flowing water and rolled along the bottom, whereas the denser and smaller grains of the darker sands were more difficult to move. The small, dense ilmenite moved the least of all. Also, small and large grains tend to sort because small grains settle beneath the large grains and support them. These two features, density and grain size, are why the middle school student could “pan” out the ilmenite and why fresh water streams and the retreating swash of ocean waves can separate the different colored sands on our beaches.