|
One problem we live with on the Oregon coast is rust. The corrosion of ferrous metals such as iron is a fact of life in coastal environments. This is not especially breaking news. Most of us know that corrosion here is rampant, and we correctly attribute it to “salt spray”. I came to the Oregon coast after living for some years in eastern Washington where the climate, though windy with blowing sand and silt, is dry and there is little salt in the sparse rainfall. As I told my friends when we were about to move, “we are trading dust for rust.” Here, my tools, which never seemed to corrode in eastern Washington, even if left out in the weather, develop a coating of rust when stored inside the garage inside tool cabinets. So, how does all this corrosive salt get into the air? The actual physics behind salt spray is elegantly described in James S. Trefil’s book, A Scientist at the Seashore (1984) and will be recapped here.
Consider bubbles and foam. As Trefil points out, three to four percent of the entire ocean surface at any one time is covered in foam generated by waves breaking out at sea and along the shore. Anyone who has watched Oregon winter storms has seen plenty of foam, especially near shore. Even the beaches may have foam piled in windrows that fly along the shoreline like the frothy heads blown off glasses of beer. Bubbles arise in the upper ocean from the churning action of the waves pumping air into the water, just like beating air into whipped cream. Pockets of air forced into the water rise to form surface bubbles. The cohesive surface tension of water molecules in the bubble allow trapped air inside to expand and force the bubble into a thin, roughly spherical film of water – somewhat like a balloon. When the upper part of the bubble pokes above the water surface, gravity tends to pull the water molecules down along its sides. The top becomes so thin that the cohesion of the remaining water molecules cannot resist the inside pressure of the air, and the bubble breaks. When it breaks, the surface tension of the water around and under the bubble causes it to snap inward and upward to fill the depression left in the water surface by the popping bubble. This surface tension is so strong that the “surface will overshoot,” propelling upward with such momentum that it breaks off one or more tiny droplets which fly away into the air. Trefil notes that you can simulate this phenomenon by tossing a pebble into a pond. The surface of the water, displaced by the stone, rushes back to fill the void and overshoots into a column of water that throws drops of water upward. trapped air inside to expand and force the bubble into a thin, roughly spherical film of water – somewhat like a balloon. When the upper part of the bubble pokes above the water surface, gravity tends to pull the water molecules down along its sides. The top becomes so thin that the cohesion of the remaining water molecules cannot resist the inside pressure of the air, and the bubble breaks. When it breaks, the surface tension of the water around and under the bubble causes it to snap inward and upward to fill the depression left in the water surface by the popping bubble. This surface tension is so strong that the “surface will overshoot,” propelling upward with such momentum that it breaks off one or more tiny droplets which fly away into the air. Trefil notes that you can simulate this phenomenon by tossing a pebble into a pond. The surface of the water, displaced by the stone, rushes back to fill the void and overshoots into a column of water that throws drops of water upward.
Foam is essentially many bubbles sticking together. However, seawater alone will not foam. If you take a jar of fresh or salt water and shake it vigorously, you will get some transient bubbles, but no foam. There is a missing essential ingredient - organic matter – which is necessary for foam formation. Organic molecules allow bubbles to stick to each other. These organic molecules come mostly from dead phytoplankton. The ocean surface has a thin film of active compounds (oils, fats, proteins, and others) about one micrometer thick called the nanolayer. The nanolayer is one part of a collection of thin layers traditionally referred to as the micro layer, home to buoyant organics, particles, microorganisms, algae, fish eggs, and larvae and various life stages of other small animals. You can see the  effects of this organic film on a calm ocean as a surface slick. Many of the organic molecules that contribute to foam are released from algae that have died and decayed. Air bubbles, formed in the turbulence of waves, collect these organics which change the water cohesion properties to let the bubbles stick together and make foam. All these bubbles in foam also burst, just as described above, releasing massive numbers of small droplets of seawater into the air, complete with both salts and organics. If you wear glasses and walk the beach on a stormy day, you will find that a greasy fog develops on your lenses which is difficult to clean off. This film is from the salts and organics contained in airborne ocean mist that collects on your glasses. The above picture illustrates this mist of droplets from churning waves and popping bubbles drifting over the south end of Netarts Bay. effects of this organic film on a calm ocean as a surface slick. Many of the organic molecules that contribute to foam are released from algae that have died and decayed. Air bubbles, formed in the turbulence of waves, collect these organics which change the water cohesion properties to let the bubbles stick together and make foam. All these bubbles in foam also burst, just as described above, releasing massive numbers of small droplets of seawater into the air, complete with both salts and organics. If you wear glasses and walk the beach on a stormy day, you will find that a greasy fog develops on your lenses which is difficult to clean off. This film is from the salts and organics contained in airborne ocean mist that collects on your glasses. The above picture illustrates this mist of droplets from churning waves and popping bubbles drifting over the south end of Netarts Bay.
These drifting droplets carry sea salts and organic material into the atmosphere to be distributed by the prevailing winds. The water in the droplets evaporates, concentrating the salts into saturated solutions and finally crystals. These particles, called aerosols, often drift inland to be deposited on our ferrous metal tools and help them rust. It is estimated that 123 million tons of sea salt world wide is aerosolized each year. Sea salt aerosols not only contribute to corrosion prob lems but also, when drifting over cities, react with atmospheric pollutants such as nitric acid and affect people’s respiratory health. lems but also, when drifting over cities, react with atmospheric pollutants such as nitric acid and affect people’s respiratory health.
Ferocious storms, like the one that hit the Oregon Coast during December 2-3, 2007 and carried winds that topped 130 miles per hour, can wreak havoc on coastal vegetation, not only by toppling trees, but also by depositing heavy loads of sea salts on leaves and branches. Most of the shore pines and Sitka spruce near the shoreline, evergreens that had kept their needles during winter, suffered severe leaf damage on their windward sides after that storm.
_______________________________________________
An interesting aside to bubbles in foam is iridescence. When you see beach foam on a sunny or slightly overcast day, look at the colors in the bubbles just before they burst. Yo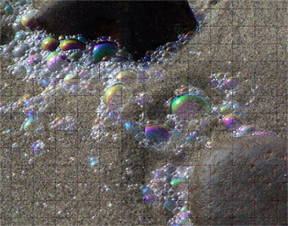 u will see in each bubble a partly reflected sun in some color of the spectrum, one purple, one green, another yellow, and so on, depending on the thickness of the bubble film. Light is reflected from both upper and lower surfaces of the film. The color has two possible explanations: one, the interference of light waves reflecting from the two surfaces, the other (and probably the more accurate) by the quantum theory of the interaction of light and matter with the “horrible name” of quantum electrodynamics, simply and entrancingly described by Richard P. Feynman (1985) in “QED – The Strange Theory of Light and Matter. I refer you to this book. u will see in each bubble a partly reflected sun in some color of the spectrum, one purple, one green, another yellow, and so on, depending on the thickness of the bubble film. Light is reflected from both upper and lower surfaces of the film. The color has two possible explanations: one, the interference of light waves reflecting from the two surfaces, the other (and probably the more accurate) by the quantum theory of the interaction of light and matter with the “horrible name” of quantum electrodynamics, simply and entrancingly described by Richard P. Feynman (1985) in “QED – The Strange Theory of Light and Matter. I refer you to this book.
|

